non coring needle
Special non-coring puncture needles for implantable port-catheter systems, consisting of surgical steel with a special punch-free bevel, tubing, closure valve and Luer-Lock connector.


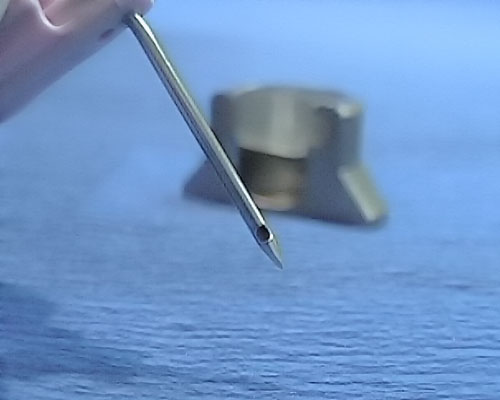


non coring needle
Special non-coring puncture needles for implantable port-catheter systems, consisting of surgical steel with a special punch-free bevel, tubing, closure valve and Luer-Lock connector.
non coring needle
Special non-coring puncture needles for implantable port-catheter systems, consisting of surgical steel with a special punch-free bevel, tubing, closure valve and Luer-Lock connector.
Indications:
Via port/catheter system continous or intermittent administration of pharmaceuticals, infusions, blood transfusions, blood-samples, parenteral nutrition, pain therapy or as central venous access perioperatively and more
Different sizes and diameters with various lengths are available depending on indications and flow rate.
Instruction for use
Non-coring puncture needles for implanted port/catheter systems needle with tubing and clamp for implanted port/catheter systems
COMPONENTS
INDICATIONS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Skin and soft tissue infections or infections of the entire portal system are possible if sterile techniques
are not used.
Warning: Puncture of the port should be performed in the supine position to prevent air embolism.
Negative pressure gradients develop in the superior vena cava in the sitting position or when the torso
is elevated. During puncture of the port, air can be sucked into the system via the needle. Therefore the
closure mechanism of the infusion tubing should be checked during puncture or when changing
syringes. When not in use, the tubing should be occluded with a screw stopper.
RECIPROCAL EFFECTS
None
GENERAL RECOMMENDATIONS
It is recommended that the needle be left in place no longer than 48 hours.
PROCEDURES
Puncture:
Warning: Needles are available in various lengths to ensure that a space remains between the skin and
the fixation plate. This is to prevent dislocation of the needle by movement of underlying tissues
such as the muscles.
a) Interruption of infusion, removal of infusion system while needle still in place
b) Removal of the needle
Important: The needle should be removed only when clamp is closed to prevent blood flow into the
system. Do not use syringes less than 10ml volume to prevent excessive pressures.
Warning: Blood deposits in the system generally indicate faulty handling, leakage, or a defective septum
inside the port.
If a thrombus in the port is suspected, the use of fibrinolytic agents such as streptokinase or urokinase
is possible. These recommendations require observation of the manufacturer’s guidelines regarding
dosages and contraindications. In pain therapy via peridural or intrathecal catheters the use of a bacterial
filter is recommended. It is prohibited to flush these systems.
Maximal flow rates (aqueous solutions) of various SFN®-Needles of various diameters and lengths to
determine infusion rate:
18 gauge, ED 1.3 mm, ID 1.0 mm, puncture length 20 mm = 1000 ml in 11 minutes
19 gauge, ED 1.1 mm, ID 0.8 mm, puncture length 20 mm = 1000 ml in 20 minutes
20 gauge, ED 0.9 mm, ID 0.6 mm, puncture length 20 mm = 1000 ml in 39 minutes
22 gauge, ED 0.7 mm, ID 0.4 mm, puncture length 20 mm = 1000 ml in 80 minutes
Single use medical product. Non-resterilizable. The needle is sterile and non-pyrogenic as long as pakkaging
is undamaged. The packed products should be stored at temperatures ranging from 5-35 °C.
Avoid direct sunlight. Do not use if expiration date on packaging is exceeded.

