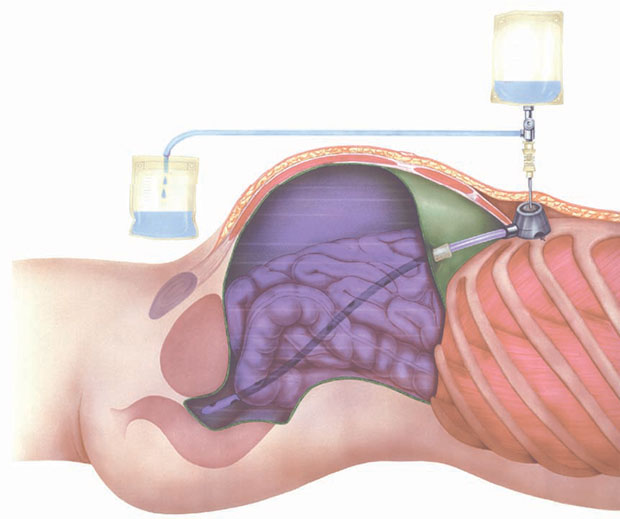
Titan ports PC (peritoneal chemotherapy) is a fully implantable, titanium PORT system providing an access facility for performing peritoneal chemotherapy. The set contains a port chamber with a self-sealing silicone membrane and a single-lumen polyurethane catheter with a cuff for connection to the port chamber and for fixing the catheter to the outlet tube. Each system also includes a special puncture needle, user instructions and patient ID documentation.
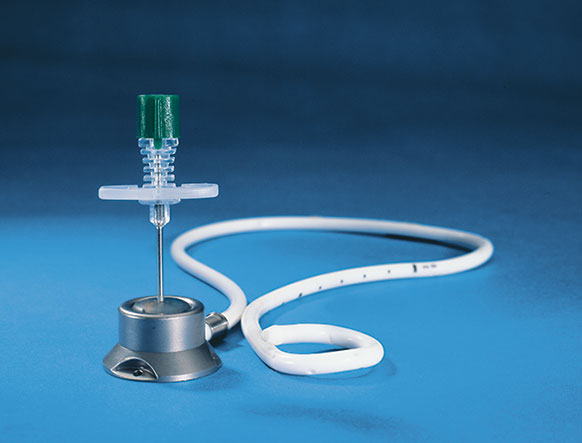
Port Chamber
The port chamber consists of hypoallergenic, biocompatible titanium. Also, this material is non-magnetic.
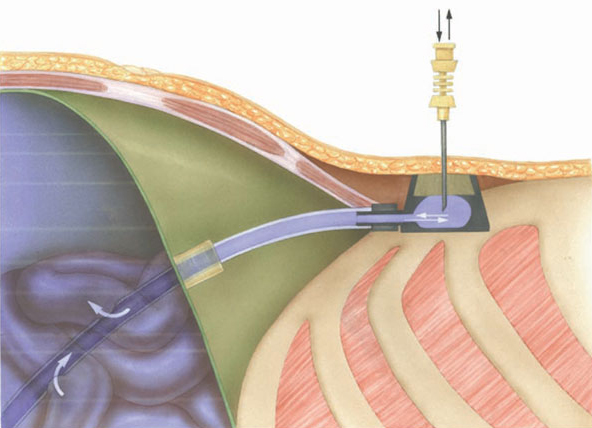
The chamber dimensions are: bottom diameter 30 mm, top diameter 21 mm, height 16 mm, weight 30 g and an outlet tube of 3.5 mm diameter in the lower part. The base plate has 3 suture openings for securing the system to the fascia. Port chamber and catheter can be disconnected and are interconnected via a squeezing mechanism with a catheter cuff via the outlet tube.
Silicone Membrane
The silicone membrane in the port (18mm diameter, height 8mm) can be punctured (up to 1000 times) with a suitable non-coring puncture needle. However, the membrane possesses high pressure stability and holds the needle securely in position.
The polyurethane catheter 13.5 F, 2.3 x 4.7 mm has 16 lateral holes at the distal end, a round tip, an overall length of 55cm, and if necessary can also be shortened at the proximal (unperforated) end and must connect via a cuff to the outlet tube of the port chamber (see illustration).
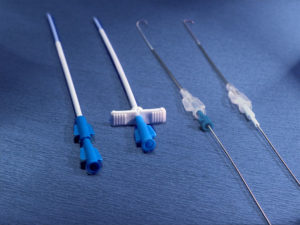
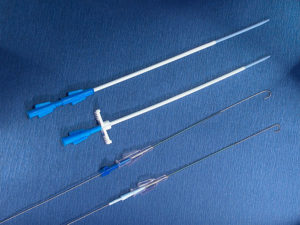
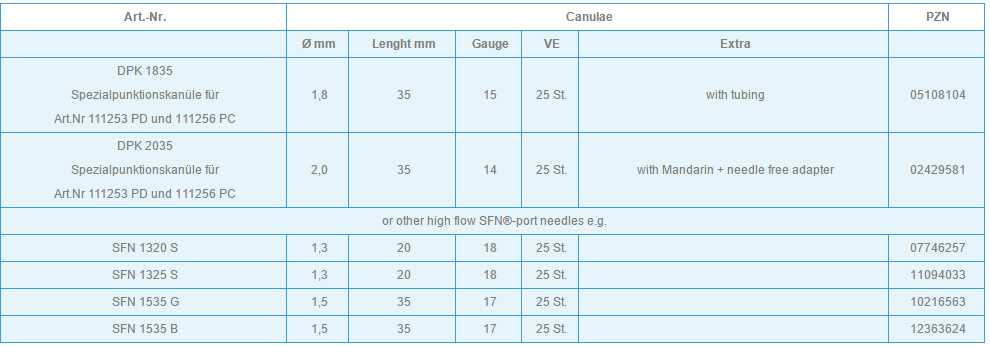
Only the special puncture needles provided must be used for puncturing the membrane (small lumen special port needles can also be used depending on indication and flow rate e.g. various size SFN port needles). However, in order to achieve high flow rates, appropriately large lumen needles are required (e.g. DPK 1630 or DPK 1625 with external diameter 1.6 mm, internal diameter 1.4 mm). These are provided with an internal mandrin, and are specially bevelled to prevent fragmentation of silicone particles and puncture defects of the membrane. Each system includes an appropriate cannula. These are available separately for further applications.
The patient ID documentation included is filled out fully by the physician who performed the implantation, and is handed to the patient who should always carry this document with him.
These user instructions should also be available to nursing staff and physicians responsible for further care.
The contents of the set are stated on the label of the double sterile blister packaging.