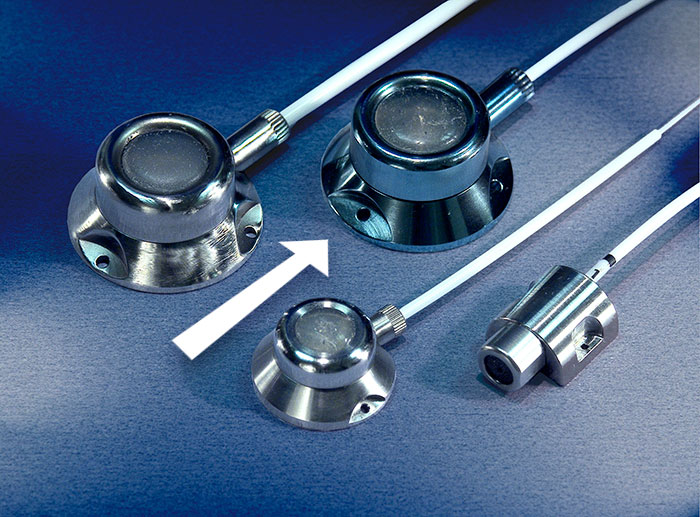
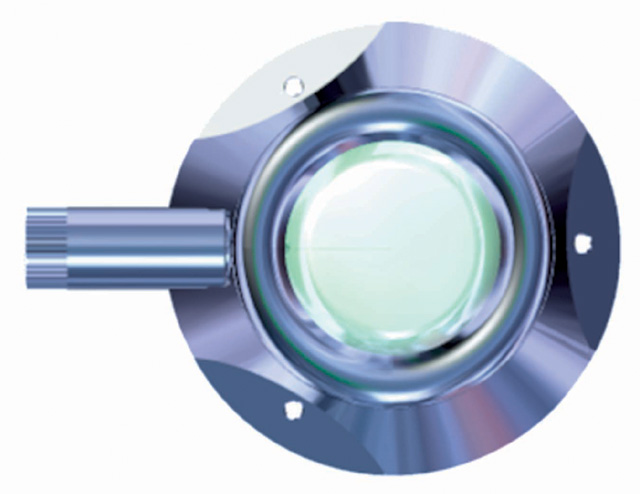
INDICATIONS
The TITAN-PORT MEDIUM Systems ensure repeated access to the vascular system for example for the following indications:
- �Llong-term treatment with cytostatic agents and other “aggressive” medications
- regional chemotherapeutic treatment of liver tumors, metastases, tumors of the head and extremities and tumors of other organs
- patients with poor peripheral veins
- infusion therapy
- parenteral nutrition
- HIV patients
- venous blood sampling
- blood transfusions *see note
- special labelled systems are appropriate to high pressure injection
In addition some systems may be used for following indications:
- TITAN-PORT A (arteriell): �for direct perfusion of organs, for inoperable hepatic metastases via the gastroduodenal artery, via the coeliac trunk for pancreatic and via the aorta and internal iliac arteries for pelvic neoplasms
- TITAN-PORT D: for dialysis venous <-> venous
- TITAN-PORT P: for pediatrics
- TITAN-PORT F: fetal
- TITAN-PORT S: �for peridural pain therapy or anesthesia
- TITAN-PORT PD: for peritoneal dialysis
- TITAN-PORT PC: for peritoneal chemotherapy
- TITAN-PORT APH: for apheresis
- TITAN-PORT AS/PT: for ascites- and peritoneal therapy
- TITAN-PORT UA: sideport
CONTRAINDICATIONS
The TITAN-PORT MEDIUM systems should not be used in:
- extremely rare cases of hypersensitivity to silicone, polyurethane or titanium
- suspected bacteremia or sepsis
- disseminated intravascular coagulation (DIC)
COMPLICATIONS and potential ADVERSE REACTIONS
The following complications or adverse reactions can occur:
- technical intraoperative complications
- tissue incompatibility
- local reactions (inflammation, edema, hematoma)
- infection
- disconnection or dislocation
- thrombosis / thromboembolism
- breakage of the catheter between the clavicle and first rib
- breakage of catheter or damage to catheter
- perforation of catheter
- drug extravasation due to improper handling of the system
- damage to neighboring tissues by the pharmaceutical agents (In the event of leakage of portal components)
INSTRUCTIONS FOR USE / PORT IMPLANTATION
Caution: During implantation sterile handling is absolutely mandatory!
- various techniques are available for implantation of port and catheter, including the Seldinger technique
- the choice depends on the surgeon’s preference
- the implantation of TITAN-PORT A/V, P and S is usually possible under local anesthesia, TITAN-PORT D, PD, PC under general anesthesia (see special instructions for use)
- recommended sites for venous catheter placement include the cephalic vein, subclavian vein, or internal and external jugular veins.
- use a 10 ml syringe and only special non-coring needles for puncturing the septum. The needles are intended for single use only.
- the needle should be inserted into the silicon membrane carefully and always vertically to avoid bending the tip.
- if the situation is unclear or thrombotic obstruction is suspected a radiographic or Doppler sonographic control is necessary.
To achieve greater flow rates the port membrane may be accessed simultaneously with up to three needles.
- a venous implantation should be flushed with 20 ml 0,9% saline solution once every 3 months if the system is not used
- an arterial implantation requires flushing once every 2 week.
- for sampling venous blood 3 ml of blood schould initially be aspirated and discarded. A minimum 20 G needle should be used and the required amount of blood aspirated slowly (to avoid falsification of the results) * note: 18 G or 19 G needle is recommended for blood transfusion! Afterwards the system must be flushed immediately with at least 20-50 ml 0,9% saline solution. During the flushing procedure the needle may be rotated in different directions to ensure unifrom rinsing of the chamber interior
- to avoid interactions between various drugs (cytostatic agents in particular) the system must be flushed with at least 10 ml 0,9% saline when repeated infusions are required
- the system must be flushed after each use. The needle is removed with gentle force
- when using needles with tube the lock schould be activated to avoid getting blood into the system
- catheter obstruction due to thrombus formation can usually be cleared by injecting Streptokinase / Urokinase (e.g. 5000 IE/ml physiologic saline with 10 ml syringe, small amounts should be injected at intervals, allowed time to take effect and the patency checked)
(The above mentioned concentrations are recommendations and must always be adapted to patient’s coagluation status as monitored by a physician. The product description of all pharmaceuticals used should always be consulted.)
- the complication rate of thrombus formation in the catheter can be reduced in patients at risk by low-dose heparinization
- in arterial portal systems, by the administration of a platelet aggregation inhibitor (e.g. acetylsalicylic acid).
CAUTION: High-pressure injections not unless patency of the system is confirmed by the physician (aspiration, manual
injection). In case of any doubt injection should be avoided!
Causes of abnormally high resistance to access needle may include
- Incorrect placement of the access needle in the portal chamber
- bending and kinking of the catheter
- fibrin deposition or thrombotic occlusion of catheter or port.
Blood deposits in the system generally indicate one of the following causes:
- leakage of the system
- defective septum
- faulty handling
INDICATIONS FOR EXPLANTATION
- the port can be removed under local anesthesia at the end of treatment (explantation is possible under local anesthesia)
- irreversible obstruction of the portal system
- breakage of catheter or damage to catheter
- membrane leakage
- poor patient compliance
- massive thrombosis of the major vessels (subclavian vein, v.cava)
- uncontrollable infection (port removal only under general anesthesia
WARNINGS
- the port should be secured to surrounding subcutaneous tissue by sutures or placed in the smallest of portal pockets in order to prevent port migration
- the catheter must be secured to the vein with a firm, but not constricting suture
- the proper connection of the catheter to the port must be confirmed
PRECAUTIONS
Sterility
Meticulous hygiene and sterile handling technique are mandatory for the safe usage of TITAN-PORT MEDIUM Systems.This includes:
- implantation in sterile OR setting
- proper disinfection of hands and skin
- sterile gloves when initiating treatment
- use of sterile products
AFTERCARE AND MONITORING
Regular treatment sessions and associated care of the port system are coincidental with monitoring of the port system.
Port systems should not be used by physicians / health care personnel not familiar with all aspects of the product and its potential complications. Complications can occur at any time during and after implantation.
CARE OF THE SYSTEM AND SPECIAL RECOMMENDATION
Before each access the correct position of the portal chamber must be checked by palpation. Any signs of infection must be excluded. Without complications the TITAN-PORT MEDIUM Systems can be used one day after implantation.
PREPARATION
- before placement, the catheter is filled with 0,9% saline solution
- the portal chamber is then punctured with the access needle, filled with 0,9% saline solution and purged of air. For this purpose the outlet pipe should be held upright when filling to allow air to escape. Use the enclosed needle.